Methylformate from CO2: an integrated process combining catalytic hydrogenation and reactive distillation†
Abstract
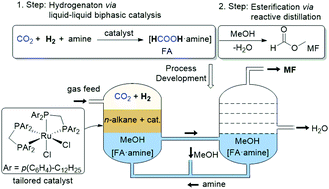
An integrated two-step process for the production of methylformate (MF) from CO2, H2 and MeOH was developed. In the first step, the hydrogenation of CO2 to a formate-amine adduct is carried out in a biphasic system comprising n-decane as the catalyst phase and MeOH as the product phase. In the second step, the resulting methanol solution containing the formate-amine adduct is subjected to reactive distillation for esterification and isolation of methylformate. The selection of the amine played an important role for devising the overall process. Whereas in the hydrogenation step basic amines work best, medium to low basic amines are preferred in the esterification step. 1,2-Dimethyl-imidazole (1,2-DMI) was identified as an effective compromise for the integration of both steps. In the hydrogenation step, a bis(diphenylphosphino)methane ligand tailored with long alkyl chains ensured effective retention of the Ru-catalyst in the non-polar phase allowing straightforward reuse of the catalyst phase. In a semi-continuous set-up, repetitive hydrogenation (8 cycles) led to a total turnover number (TTON) of 38 000 at an average turnover frequency (TOF) of 1400 h−1 with a cumulative catalyst leaching of only 1.4 mol% for P and 2.0 mol% for Ru. Reactive distillation was demonstrated in a continuously operated rectification unit leading to the isolation of MF at the head of the column with a purity of 91.5%.
- This article is part of the themed collection: 2019 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...