Achmatowicz rearrangement enables hydrogenolysis-free gas-phase synthesis of pentane-1,2,5-triol from furfuryl alcohol
Abstract
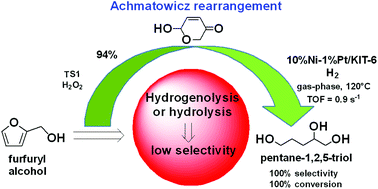
Highly efficient synthesis of pentane-1,2,5 triol, a promising member of biorenewable C5 alcohols, has been achieved by gas-phase hydrogenation of the Achmatowicz intermediate derived from furfuryl alcohol. The hydrogenation was carried out on monocomponent or bicomponent Ni and/or Pt modified mesoporous silica catalysts. The process features the absence of hydrogenolysis of the furan ring and rendered 100% selectivity together with additional green chemistry benefits, such as mild and simpler solvent free technology that operates at atmospheric pressure. The bicomponent Ni/Pt modified mesoporous silica catalysts exhibited the highest catalytic activity, with 10Ni1Pt/KIT-6 being the most active, providing up to 100% conversion.


 Please wait while we load your content...
Please wait while we load your content...