ReGreen SPPS: enabling circular chemistry in environmentally sensible solid-phase peptide synthesis†
Abstract
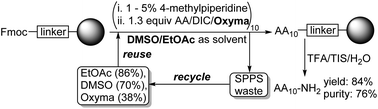
Aiming at greener, scalable methods for cost-efficient peptide synthesis a solid-phase peptide synthesis (SPPS) protocol adhering to the principles of circular chemistry was developed. The method we term ReGreen SPPS utilizes industrially viable polystyrene/divinylbenzene (PS/DVB) resins as polymer supports, inexpensive ethyl acetate (EtOAc) and dimethylsulfoxide (DMSO) as solvents, is synthetically efficient in terms of equivs of amino acids (AAs) in couplings and amounts of base in Fmoc removals and afforded a model difficult 10-mer peptide in 84% yield and 76% purity. A facile process for reagent and solvent recycling of the waste stream from a SPPS process using greener solvents was developed by a simple protocol for the recovery of EtOAc and DMSO (solvents) as well as the coupling agent ethyl (hydroxyimino)cyanoacetate (Oxyma). Compared to the conventional (DMF) SPPS the complete E factor (cEF) for the ReGreen SPPS method was lowered from ∼2200 to ∼500 while the total solvent cost was reduced ca two-fold (to 151 EUR per AA cycle on 1 mol scale). Further, the recycled EtOAc, DMSO and Oxyma were used in a model SPPS coupling vs. the corresponding virgin materials in which the obtained crude peptides were undistinguishable, thereby proving that the recycled chemicals can be reused without detrimental effects on the synthesis. The oxidation susceptible AA Cys was assessed in Oxyma/diisopropylcarbodiimide (DIC) mediated couplings using the greener DMSO/EtOAc vs. conventional dimethylformamide (DMF) as solvents. A crude model 6-mer was synthesized in a higher yield and purity in DMSO/EtOAc than in DMF which renders ReGreen SPPS not only the first SPPS in greener solvents on a PS/DVB resin that adopts the principles of circular chemistry but also provides a method that represent a chemoselectivity improvement over conventional peptide synthesis.
- This article is part of the themed collection: Measuring Green Chemistry: Methods, Models, and Metrics


 Please wait while we load your content...
Please wait while we load your content...