Continuous flow solvent free organic synthesis involving solids (reactants/products) using a screw reactor†
Abstract
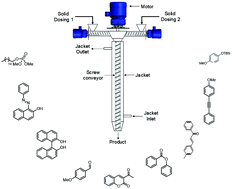
Here we report for the first-time various organic transformations such as aldol condensation, oxidation, nucleophilic substitutions, protection, acylations and coupling reactions using a mechanochemical approach at a controlled temperature using a single synthesis platform. Almost minimal solvents or solvent-free conditions are used, making it a very efficient and clean synthesis of various products. A jacketed screw reactor when operated at different temperatures (0 °C to 160 °C) and over a range of rotation speeds for changing the residence time (15 s–300 s) helped to achieve maximum conversion. This approach is also extended to the synthesis using substrates having different substitutions, heterocycles and steric hindrance.


 Please wait while we load your content...
Please wait while we load your content...