Merrifield resin-supported quinone as an efficient biomimetic catalyst for metal-free, base-free, chemoselective synthesis of 2,4,6-trisubstituted pyridines†
Abstract
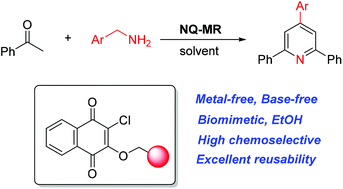
Metal-free, base-free, biomimetic and chemoselective synthesis of 2,4,6-trisubstituted pyridines was developed under mild conditions for the first time. The heterogeneous biomimetic catalyst – recoverable Merrifield resin-supported quinone – was fully characterized by Fourier transform infrared spectroscopy (FT-IR), X-ray photoelectron spectrometry (XPS) and energy dispersive X-ray spectroscopy (EDX). This supported quinone catalyst exhibited excellent catalytic reactivity for chemoselective synthesis of 2,4,6-trisubstituted pyridines, providing an efficient and green method for the synthesis of pyridine derivatives under mild conditions. Mechanistic investigations were conducted to gain insights into the heterogeneous biomimetic catalyst as well as the resulting transformation. The successful capture of intermediates offered direct and clear evidence for the proposed mechanism.


 Please wait while we load your content...
Please wait while we load your content...