Cercosporin-bioinspired selective photooxidation reactions under mild conditions†
Abstract
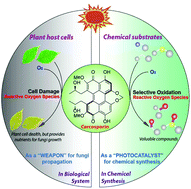
The development of an efficient system for selective oxidation of organic compounds to generate more valuable compounds with molecular oxygen is a significant challenge in industrial chemistry. Bioinspired by the ability of naturally occurring perylenequinonoid pigments (PQPs) to generate reactive oxygen species (ROS) upon photoirradiation, here we report that cercosporin, one of the perylenequinonoid pigments, can function as a cost-effective and environmentally friendly photocatalyst for a wide range of selective oxidations, including benzylic C–H bonds to carbonyls, amines to aldehydes, and sulfides to sulfoxides. All of the representative reactions proceeded smoothly with high efficiency under mild conditions. Owing to the use of inexpensive metal-free visible light-driven photocatalyst produced from microbial fermentation with cheap glucose as the starting material and the ease of handling, we expect that this developed method will be particularly attractive for many more applications in synthetic transformation.


 Please wait while we load your content...
Please wait while we load your content...