Metal- and oxidant-free electrochemical synthesis of sulfinic esters from thiols and alcohols†
Abstract
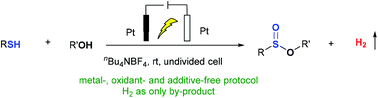
An efficient and eco-friendly electrochemical synthesis of various sulfinic esters from thiols and alcohols via sequential S–H/S bond cleavage and double S–O bond formation under mild reaction conditions has been developed. Stoichiometric oxidants, metal catalysts, activating agents and even added bases were avoided in this method, and the only by-product generated from this reaction was dihydrogen gas which could serve as a green source of energy. Various functional groups are compatible with this green protocol which can be easily conducted on a gram-scale.


 Please wait while we load your content...
Please wait while we load your content...