Iron-catalysed 1,2-acyl migration of tertiary α-azido ketones and 2-azido-1,3-dicarbonyl compounds†
Abstract
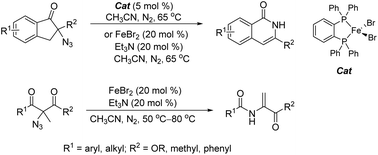
Iron-catalysed 1,2-acyl migration of tertiary α-azido ketones and 2-azido-1,3-dicarbonyl compounds provides a simple and atom-economical approach toward enamides and isoquinolones. This paper reports two catalyst systems for these transformations which employ iron(II) complexes [Fe(dpbz)]Br2 (dpbz = 1,2-bis(diphenylphosphino)benzene) and FeBr2/Et3N, respectively. [Fe(dpbz)]Br2 was found to be highly effective at converting 2-azido-2,3-dihydro-1H-inden-1-ones to isoquinolones. The reagent combination of FeBr2/Et3N, on the other hand, exhibited a broader catalytic scope, owing to the beneficial effect of Et3N. This latter catalyst system enables 2-azido-2-methyl-1,3-dicarbonyl compounds to be converted to the corresponding enamides under mild conditions in good yields.


 Please wait while we load your content...
Please wait while we load your content...