Scale-up biopolymer-chelated fabrication of cobalt nanoparticles encapsulated in N-enriched graphene shells for biofuel upgrade with formic acid†
Abstract
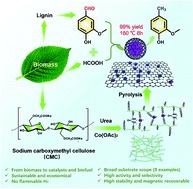
Exploring both high-performance catalytic materials from non-edible lignocellulosic biomass and selective hydrodeoxygenation of bioderived molecules will enable value-added utilization of renewable feedstocks to replace rapidly diminishing fossil resources. Herein, we developed a scale-up and sustainable method to fabricate gram-quantities of highly dispersed cobalt nanocatalysts sheathed in multilayered N-doped graphene (Co@NG) by using a biomacromolecule carboxymethyl cellulose (CMC) as a raw material. The ionic gelation of CMC, urea and Co2+ ions leads to uniform dispersion and chelation of different species, consequently resulting in the formation of highly distributed Co nanoparticles (NPs) (10.91 nm) with N-enriched graphene shells in the solid-state thermolysis process. The usage of urea as a non-corrosive activation agent can introduce a porous belt-like nanostructure and abundant doped nitrogen. Among all the prepared catalysts in this work, the optimized Co@NG-6 with the largest specific surface area (627 m2 g−1), the most and strongest basic sites, and the highest proportion of pyridinic-N (37.6%) and mesopores exhibited excellent catalytic activity (99% yield of 2-methoxy-p-cresol) for base-free transfer hydrodeoxygenation (THD) of vanillin using bioderived formic acid (FA) as a H source at 160 °C for 6 h. The poisoning tests and electron paramagnetic resonance (EPR) spectra verified that the strong interaction between N atoms and encapsulated Co NPs provided synergistic effects, which were essential for the outstanding catalytic performance of Co@NG-6. The deuterium kinetic isotope effect study clearly demonstrated that the formation of Co-H−via β-hydride elimination and protonation was the rate-determining step, and protic N–H+ and hydridic Co-H− were considered to be active intermediate species in the THD reaction. Furthermore, Co@NG-6 was highly stable for recycling owing to the graphene shells preventing Co NPs from corrosion and aggregation.


 Please wait while we load your content...
Please wait while we load your content...