A comparative study of secondary depolymerization methods on oxidized lignins†
Abstract
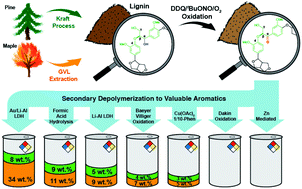
Selective oxidation of lignin's β-aryl ether units combined with secondary chemical treatment for depolymerization can generate valuable oxygen-rich aromatics. Although there have been many reports of the successful oxidative depolymerization of lignin, an accurate assessment of the merits of each method is hampered by the wide array of lignins used. Here, we test a selection of literature methods for secondary lignin depolymerization using a common set of lignin substrates. In an initial step, the lignins were oxidized using 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ)/tert-butyl nitrite (tBuONO)/O2. The oxidized lignins were then subjected to a variety of depolymerization methods, the yield of aromatic monomers being quantified and compared to lignin depolymerized using an Au/Li-Al LDH catalyst followed by hydrolysis without prior Cα-OH oxidation. The Au/Li-Al LDH system gave the highest monomer yield for the untreated lignins, moreover, for DDQ-oxidized lignins, the Au/Li-Al LDH method produced similar monomer yields with high selectivity towards aromatic acids and aldehydes.


 Please wait while we load your content...
Please wait while we load your content...