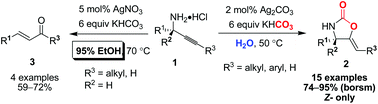
Silver(i)-catalysed carboxylative cyclisation of primary propargylic amines in neat water using potassium bicarbonate as a carboxyl source: an environment-friendly synthesis of Z-5-alkylidene-1,3-oxazolidin-2-ones†
Abstract
Herein, we report a mild and environment-friendly synthesis of Z-5-alkylidene-2-oxazolidinones in neat water, using a low loading (2 mol%) of silver carbonate as a catalyst. Instead of pressurised gaseous carbon dioxide, potassium bicarbonate was used as the source of carboxyl. An interesting solvent effect and a C–N cleavage side reaction with a 6-endo-dig mechanism are also discussed.


 Please wait while we load your content...
Please wait while we load your content...