Flow-based enzymatic synthesis of melatonin and other high value tryptamine derivatives: a five-minute intensified process†
Abstract
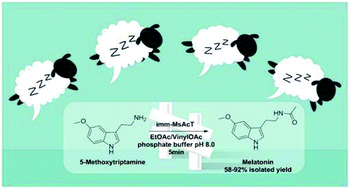
To increase the uptake of biocatalytic processes by industry, it is essential to demonstrate the reliability of enzyme-based methodologies directly applied to the production of high value products. Here, a unique, efficient, and sustainable enzymatic platform for the multi-gram synthesis of melatonin, projected to generate around 1.5 billion U.S. dollars worldwide by 2021, and its analogues was developed. The system exploits the covalent immobilization of MsAcT (transferase from Mycobacterium smegmatis) onto agarose beads increasing the robustness and longevity of the immobilized biocatalyst. The fully-automated process deriving from the integration between biocatalysis and flow chemistry is designed to maximize the overall yields (58–92%) and reduce reaction times (5 min), overcoming the limitation often associated with bioprocesses and bridging the gap between lab scale and industrial production.


 Please wait while we load your content...
Please wait while we load your content...