Reactions of α-haloacroleins with azides: highly regioselective synthesis of formyl triazoles†
Abstract
A general metal-free route to 1,4-disubstituted and 1,4,5-trisubstituted 1,2,3-triazoles was developed. α-Haloacroleins reacted with organic azides in a DMSO/H2O mixture solvent at room temperature to produce 1,4-disubstituted triazoles (up to 99%) with exclusive regioselectivities. This protocol is convenient and scalable with a broad substrate scope including aliphatic and aromatic azides. The resulting triazoles exhibited an aldehyde group at the C4 position and demonstrated synthetic utilizations. One 1,2,3-triazole compound containing diastereotopic protons was also identified.
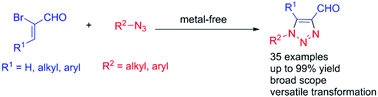


 Please wait while we load your content...
Please wait while we load your content...