The cooperative effect of Lewis and Brønsted acid sites on Sn-MCM-41 catalysts for the conversion of 1,3-dihydroxyacetone to ethyl lactate†
Abstract
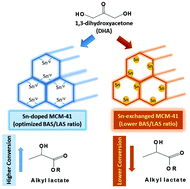
Lactic acid and alkyl lactates are widely applied in the production of food, cosmetics, pharmaceuticals, organic synthesis and biodegradable polymers. They can be prepared via one-pot synthesis from renewable trioses, such as dihydroxyacetone (DHA). Brønsted–Lewis bifunctional solid acids (BAS & LAS) can promote the reaction via a two-step cascade reaction mechanism. BAS catalyses the dehydration of DHA, resulting in the formation of pyruvaldehyde (PA) via the rearrangement of the enol form. Upon alcohol addition, PA can be converted to the desired alkyl lactates at LAS or to pyruvaldehyde hemiacetal (PAHA) at strong BAS. The density and strength control of Brønsted acid sites (BAS) and Lewis acid sites (LAS) and the optimization of their cooperation are essential for the efficient conversion of trioses to the target products. Here, we prepared a series of Sn-containing mesoporous MCM-41 catalysts with various BAS/LAS ratios by room temperature techniques. Sn-doped [Si]MCM-41 having a lower BAS/LAS ratio in this research shows a high initial selectivity to ethyl lactate (EL) and similar EL yield in 6 hours as the reported best Sn catalyst Sn-grafted [Si]MCM-41/carbon network materials in DHA conversion. A relatively large density of LAS in Sn-doped [Si]MCM-41 causes a fast conversion of PA to EL, while the overall yield has been limited by the BAS density for the DHA conversion. New H-form [Sn]MCM-41, having a suitable density of LAS and weak BAS and an optimized BAS/LAS ratio, provides a 100% yield of ethyl lactate in the catalytic conversion of DHA in ethanol within 30 min, showing a superior performance hitherto.


 Please wait while we load your content...
Please wait while we load your content...