Engineering faster transglycosidases and their acceptor specificity†
Abstract
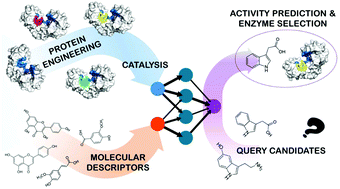
Transglycosidases are enzymes that have the potential to catalyze the synthesis of a wide range of high-value compounds starting from biomass-derived feedstocks. Improving their activity and broadening the substrate range are important goals to enable the widespread application of this family of biocatalysts. In this work, we engineered 20 mutants of the rice transglycosidase Os9BGlu31 and evaluated their catalysis in 462 reactions over 18 different substrates. This allowed us to identify mutants that expanded their substrate range and showed high activity, including W243L and W243N. We also developed double mutants that show very high activity on certain substrates and exceptional specificity towards hydrolysis, such as L241D/W243N. In order to guide a more general use of Os9BGlu31 variants as transglycosylation catalysts, we built cheminformatics models based on topological descriptors of the substrates. These models showed useful predictive potential on the external validation set and are allowing the identification of efficient catalytic routes to novel phytohormone and antibiotic glucoconjugates of interest.


 Please wait while we load your content...
Please wait while we load your content...