Efficient and catalyst free synthesis of acrylic plastic precursors: methyl propionate and methyl methacrylate synthesis through reversible CO2 capture†
Abstract
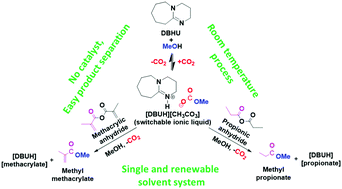
Methyl propionate (MP) and methyl methacrylate (MMA) are considered as industrially important precursors for large-scale acrylic plastic production. The existing industrial synthetic protocols for these precursors utilize expensive catalysts accompanied by toxic and explosive gases such as carbon monoxide, ethylene and hydrogen. Herein, we for the first time report highly selective, catalyst-free and room temperature synthesis of MP and MMA precursors through a reversible CO2 capture approach involving an organic superbase. In short, initially an equimolar mixture of an organic superbase 1,8-diazabicyclo-[5.4.0]-undec-7-ene (DBU) and methanol was reversibly reacted with molecular CO2 and the obtained switchable ionic liquid, [DBUH][MeCO3], further reacted with an equivalent amount of propionic anhydride or methacrylic anhydride to form MP or MMA, respectively. These reactions were accomplished in different solvents such as DMSO and methanol, and in the case of methanol, separation of reaction products occurs from in situ formed DBU derivatives such as [DBU][propionate] or [DBU][methacrylate]. In the case of both MP and MMA syntheses, after the use of methanol as a solvent, good recovery of the alcoholic solution of esters was achieved where 85% and 92% yields of MP and MMA were obtained, respectively. Molecular DBU was recovered from its propionate and methacrylate salts using NaCl saturated alkaline solution. Furthermore, the recovered MMA with methanol was polymerised to poly-MMA using a benzoyl peroxide induced free radical polymerisation process. The synthesis and separation of MP or MMA and the recovery of DBU were monitored by NMR analysis. Hence, unlike DMSO, methanol not only functioned as a reagent in CO2 capture and as a solvent medium in MP, MMA and poly-MMA syntheses but also assisted in the recovery of DBU from the reaction mixture. Most importantly, here we present a more efficient, safer and single solvent based alternative synthetic approach for the synthesis of acrylic plastic precursors MP or MMA compared to existing industrial methods. Also, no toxic or expensive catalysts were required.


 Please wait while we load your content...
Please wait while we load your content...