Synthesis, characterization, and water-degradation of biorenewable polyesters derived from natural camphoric acid†
Abstract
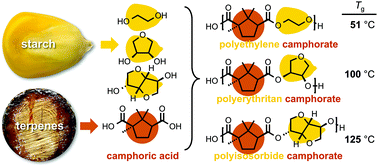
Camphoric acid, an inexpensive and biorenewable diacid derived from the terpene (+)-camphor, was copolymerized with a variety of diols to afford polyesters with glass transition temperatures (Tg) ranging from −16 °C to 125 °C. Polyethylene camphorate (PEC, 51 °C), polyerythritan camphorate (100 °C), and polyisosorbide camphorate (125 °C) exhibited Tg values matching or excelling those of important commercial polymers. Agitation of PEC in deionized water for 14 days dramatically degraded the polymer from Mn = 20 200 to Mn < 600. Incremental replacement of terephthalic acid with camphoric acid led to a series of polyethylene (camphorate/terephthalate) analogues with increased biobased content and Tg values (71 to 41 °C) that were diminished, but still competitive with that of polylactic acid (PLA).


 Please wait while we load your content...
Please wait while we load your content...