Aromatics from lignin through ultrafast reactions in water†
Abstract
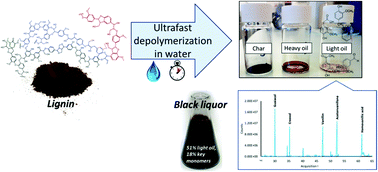
Nowadays, the valorization of lignin, the major natural source of aromatics, is a challenge for the scientific community. In this study, kraft lignin was effectively converted into aromatic monomers via ultrafast depolymerization in hot and pressurized water. At reaction times below 500 ms, it was possible to avoid char formation originating from undesirable condensation reactions by accurately controlling the reaction time. In alkaline medium, the reaction was optimum at 386 °C and 300 ms with a light oil yield of 60% and concentration of key compounds such as guaiacol, creosol, vanillin and acetovanillone of around 20% w/w. The total aromatic monomeric yield based on lignin was 10.5% w/w. The char formation at this point was surprisingly low (4% w/w). Analysis and quantification of the products allowed the evolution of the different reaction steps to be identified and a plausible mechanism for the depolymerization and repolymerization stages to be proposed. Furthermore, it was proven that the proposed technology is equally effective to directly treat industrial black liquors with a yield higher than 50% to light oil, containing guaiacol (2.7%), syringol (3.0%) and syringaldehyde (7.3%) as the main monomers.
- This article is part of the themed collection: Green Biorefinery Technologies based on Waste Biomass


 Please wait while we load your content...
Please wait while we load your content...