Rhodium(i)-catalyzed Pauson–Khand-type reaction using formic acid as a CO surrogate: an alternative approach for indirect CO2 utilization†
Abstract
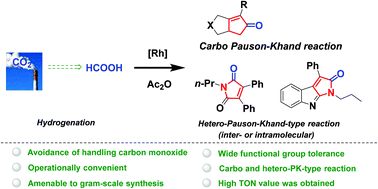
Formic acid is found to be an ideal CO surrogate for the rhodium(I)-catalyzed Pauson–Khand-type (PK-type) reaction of various substituted 1,6-enynes to afford bicyclic cyclopentenones in moderate to good yields. High TON value of up to 263 and good results in the gram-scale experiment were also obtained, demonstrating the efficacy of this methodology. In addition, heterocyclic molecules of pharmaceutical importance were also furnished via inter- or intra-molecular hetero-PK-type reactions, further broadening the application of current strategy. In this protocol, formic acid was utilized as a bridging molecule for the conversion of CO2 to CO, since formic acid is manufactured via catalytic hydrogenation of CO2 and releases CO in the presence of acetic anhydride readily. Therefore, this methodology represents a green and indirect approach for chemical valorization of CO2 in the preparation of value-added compounds.


 Please wait while we load your content...
Please wait while we load your content...