Ligand-free palladium catalyzed Ullmann biaryl synthesis: ‘household’ reagents and mild reaction conditions†
Abstract
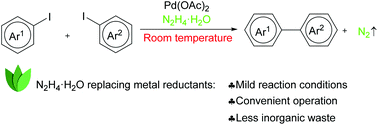
A palladium catalysed Ullmann biaryl synthesis has been developed using hydrazine hydrate as the reducing reagent at room temperature. The combination of Pd(OAc)2 and hydrazine hydrate works smoothly for the coupling of both electron-rich and electron-deficient aryl iodides, as well as hetero-aryl iodides, leading to a wide range of biaryls in good to excellent yields. The reaction requires only 1 mol% Pd(OAc)2 and the in situ generated palladium naoparticles are found to be active catalysts.


 Please wait while we load your content...
Please wait while we load your content...