Selective synthesis of citrus flavonoids prunin and naringenin using heterogeneized biocatalyst on graphene oxide†
Abstract
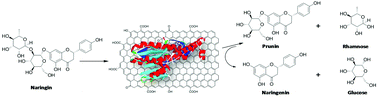
Production of citrus flavonoids prunin and naringenin was performed selectively through the enzyme hydrolysis of naringin, a flavonoid glycoside abundant in grapefruit wastes. To produce the monoglycoside flavonoid, prunin, crude naringinase from Penicillium decumbens was purified by a single purification step resulting in an enzyme with high α-rhamnosidase activity. Both crude and purified enzymes were covalently immobilized on graphene oxide. The activity of the immobilized enzymes at different pH levels and temperatures, and the thermal stability were determined and compared with those exhibited by the free naringinases using specific substrates: p-nitrophenyl-β-D-glucoside (Glc-pNP) and p-nitrophenyl-alpha-L-rhamnopyranoside (Rha-pNP). The crude and purified naringinase supported on GO were tested in the hydrolysis of naringin, giving naringenin and prunin, respectively, in excellent yields. The supported enzymes can be reused many times without loss of activity. The naringinase stabilized on GO has high potential to produce the valuable citrus flavonoids prunin and naringenin.


 Please wait while we load your content...
Please wait while we load your content...