Efficient, one-step, cascade synthesis of densely functionalized furans from unprotected carbohydrates in basic aqueous media†
Abstract
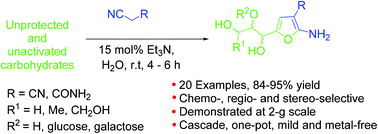
Condensation of unprotected carbohydrates with malononitrile or malonamide nitrile in basic aqueous media gave in one-pot densely functionalized polyhydroxyalkyl 2-amino-3-furanonitriles and polyhydroxyalkyl 2-amino-3-furanocarboxamides in excellent 84–98% yields. Disaccharides afforded novel furano-glycosides. Based on the rate of formation of the furans, the ability of the tested bases to catalyze the reaction was concluded as Et3N > DBU > K2CO3 > NaOMe. The reaction mechanism involved the formation of bicyclic furan-furan and furan-pyran intermediates observed for the first time. The reaction proceeded through a cascade mechanism involving Knoevenagel condensation, Oxo-Micheal addition, Thorpe-Ziegler type reaction and ring-opening-induced aromatization. This operationally simple and metal-free reaction proceeded with exclusive chemo-, regio-, and stereo-selectivities under environmentally benign conditions. As demonstrated on a 2 g scale, it holds promise for application in the large-scale synthesis of important intermediates such as densely functionalized furfural.


 Please wait while we load your content...
Please wait while we load your content...