A micellar catalysis strategy applied to the Pd-catalyzed C–H arylation of indoles in water†
Abstract
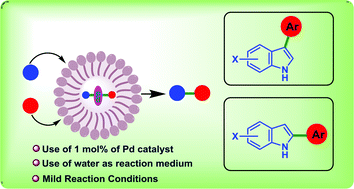
The selective control over multiple competing C–H sites would enable straightforward access to functionalized indoles. In this context, we report here a modular and selective C–H arylation of indoles following the micellar catalysis approach using the third generation “designer” surfactant SPGS-550-M in the presence of 1 mol% of [(cinnamyl)PdCl]2 under mild conditions. Thus, access to high value C-arylated (C-3 and C-2) indoles was achieved fulfilling the “triple bottom line philosophy” of green chemistry. The nature of the phosphine ligand was found to be critical for achieving site-selectivity, DPPF and DPPP being the most effective in promoting the arylation at C3–H and C2–H, respectively. The reaction is scalable and offers high chemo- (C vs. N) and regio-selectivity (C-3 vs. C-2) with a wide range of functional group tolerance. The surfactant aqueous solution can be recycled and reused without compromising on product yields.


 Please wait while we load your content...
Please wait while we load your content...