Electrodeposition of indium from the ionic liquid trihexyl(tetradecyl)phosphonium chloride†
Abstract
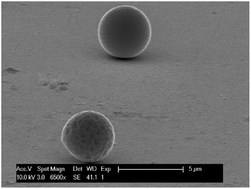
The electrochemical behavior of indium in the ionic liquid trihexyl(tetradecyl)phosphonium chloride (Cyphos IL 101) was studied. Cyphos IL 101 first had to be purified, as the impurities present in commercial Cyphos IL 101 interfered with the electrochemical measurements. Electrochemical deposition of indium metal from this electrolyte occurs without hydrogen evolution, increasing the cathodic current efficiency compared to deposition from water and avoiding porosity within the deposited metal. Indium(III) is the most stable oxidation state in the ionic liquid. This ion is reduced in two steps, first from indium(III) to indium(I) and subsequently to indium(0). The high thermal stability of Cyphos IL 101 allowed the electrodeposition of indium at 120 °C and 180 °C. At 180 °C indium was deposited as liquid indium which allows for the easy separation of the indium and the possibility to design a continuous electrowinning process. On molybdenum, indium deposits as liquid droplets even below the melting point of indium. This was explained by the combination of melting point depression and undercooling. The possibility to separate indium from iron and zinc by electrodeposition was tested. It is possible to separate indium from zinc by electrodeposition, but iron deposits together with indium.


 Please wait while we load your content...
Please wait while we load your content...