Kinetic understanding of the effect of Na and Mg on pyrolytic behavior of lignin using a distributed activation energy model and density functional theory modeling
Abstract
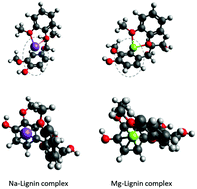
Alkali and alkaline earth metals (AAEMs) naturally occurring in most lignocellulosic biomass have strong catalytic reactivity during biomass depolymerization. Considering the fact that only a small amount of AAEMs can dramatically change the thermal pathway of each biomass component, an in-depth understanding of the effect of AAEMs on pyrolysis kinetics is necessary to develop the catalytic and thermal routes for effective lignin depolymerization. This work investigated the pyrolysis kinetics of Na and Mg metal-infused lignin using a distributed activation energy model (DAEM) to analyze experimental pyrolysis data. We also performed computational modeling of lignin decomposition using density functional theory (DFT) and Bader's atoms-in-molecules (AIM) analysis to understand the bond properties and calculate the bond length of the aryl–ether linkage in the lignin model dimer and the stabilized energy level in the presence of Na and Mg. Quantum mechanical calculation allowed us to understand how the metal ions interact at the molecular level with the lignin structure. Along with computational studies, kinetic parameters including the distribution curve of the activation energy, f(E), and the activation energy dependent frequency factor, k0, were obtained. The peak of the f(E) curve was centered at 180 kJ mol−1 for the control lignin, which increased up to 208 kJ mol−1 with the infusion of Mg but decreased to 145 kJ mol−1 with Na treatment. DFT calculations on lignin model compounds identified the most stable metal–lignin complex, in which metals can bind the O(Cβ) and O(methoxy) forming a stable half-sandwich structure. Although Mg has a stronger catalytic effect elongating the dissociating bond, Cβ–O, the Mg–lignin complex dramatically reduces the stabilized energy, which brings strong recalcitrance to lignin decomposition. These results provide both kinetic parameter level and molecular level understanding of the effect of AAEMs on lignin pyrolysis, allowing the development of more useful pyrolysis models.


 Please wait while we load your content...
Please wait while we load your content...