Integrated metabolomic and proteomics profiling reveals the promotion of Lactobacillus reuteri LR1 on amino acid metabolism in the gut–liver axis of weaned pigs
Abstract
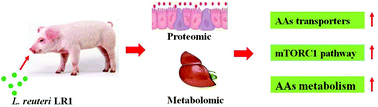
Lactobacillus reuteri LR1 improved growth performance of weaned pigs in our previous study. The objective of this study was to reveal effects of L. reuteri LR1 on amino acid (AA) metabolism in weaned pigs and its underlying mechanism using metabolomic and proteomics methods. Weaned pigs were fed a basal diet (CON) or the basal diet supplemented with 5 × 1010 cfu kg−1L. reuteri LR1 (LR1) for a 14 d period. Untargeted metabolomic analysis of the liver showed that LR1 up-regulated 33 metabolites and most of them were related to AA metabolism. Quantitative proteomics found that differential proteins were mainly involved in a metabolic process in the ileal mucosa of LR1 vs. CON. Integrated metabolomic and proteomics analysis showed that the LR1's enhancement of AA metabolism in the gut–liver axis is mediated by the up-regulated intestinal AA transporters in the pathway of protein digestion and absorption. Moreover, qPCR results confirmed that LR1 increased (p < 0.05) mRNA abundances of AA transporters (PepT1, EAAT3, rBAT, B0AT1, and b0,+AT) in the ileal mucosa compared with CON. Furthermore, western blot analysis showed that LR1 activated the mammalian target of the rapamycin complex 1 (mTORC1) signaling pathway by increasing the phosphorylation of S6 and 70S6K1 in the gut–liver axis of weaned pigs. Together, these data indicated that dietary supplemented LR1 enhanced AA metabolism by up-regulating intestinal AA transporter expression and activating the mTORC1 signaling pathway in the gut–liver axis of weaned pigs.


 Please wait while we load your content...
Please wait while we load your content...