Identification and molecular mechanism of antithrombotic peptides from oyster proteins released in simulated gastro-intestinal digestion†
Abstract
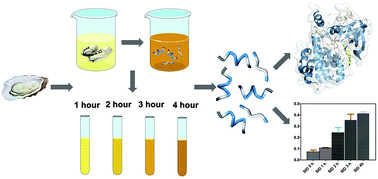
In this study, oyster (Crassostrea gigas) proteins were digested under in vitro gastrointestinal conditions to screen potential antithrombotic peptides. The sequences of the released peptides in the intestinal digestion phase were identified by ultra-performance liquid chromatography coupled to quadrupole time-of-flight MS (UPLC-Q-TOF-MS/MS). According to the antithrombotic activity analysis, the inhibitory ratio of oyster peptides showed an increasing trend, reaching up to 35.80% for a digestion period of 4 h. The APTT (activated partial thromboplastin time) and TT (thromboplastin time) were increased by oyster peptides for human serum in vitro. Oyster peptides showed a competitive inhibition effect on thrombin, based on Lineweaver–Burk plot analysis. Molecular docking between the antithrombotic peptides and thrombin (PDB: 5ZJY) was conducted using Discovery Studio 2017. Potential inhibitors against thrombin and the mechanism of antithrombotic activity were predicted using the algorithm of CDOCKER. There are fourteen potential antithrombotic peptides, whose affinity with thrombin is higher than that of hirudin, as indicated by the “-CDOCKER energy” score (181.491). Peptide LSKEEIEEAKEV is similar in sequence to thrombin inhibitors. The binding sites of potential antithrombotic peptides against thrombin at the S1 pocket were compared with hirudin variant-2 (GDFEEIPEEYLQ). In addition, the peptides containing the RG/RGD sequence were identified, which can be hydrolyzed by thrombin as a substrate. Consequently, the oyster peptides released in simulated gastrointestinal digestion probably inhibit thrombin in two ways, not only as the inhibitor against the active site, but also as the substrate of thrombin. These results maybe be attributed to the potentially strong antithrombotic activity in the human digestive system.

 Please wait while we load your content...
Please wait while we load your content...