The purification, identification and bioactivity study of a novel calcium-binding peptide from casein hydrolysate
Abstract
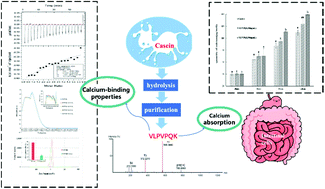
In this study, a novel calcium-binding peptide from casein hydrolysate was purified using reversed-phase high performance liquid chromatography and sequenced by high-performance liquid chromatography-mass spectrometry (MS)/MS. The amino acid sequence of the calcium-binding peptide was identified as VLPVPQK (N- to C-terminal, MW = 779.4960 Da). The calcium binding characteristics of VLPVPQK were further investigated using UV absorption spectroscopy, zeta potential and isothermal titration calorimetry (ITC). The results showed that VLPVPQK has a strong calcium binding activity (129.46 mg g−1), 312% higher than that of 3-hour enzymatic hydrolysates. VLPVPQK could chelate calcium with a 1 : 3 stoichiometry, causing a decrease in the positive charge of the peptide–Ca2+ complex. Furthermore, VLPVPQK could effectively enhance calcium transport and absorption in a concentration-dependent manner in Caco-2 cell monolayers, suggesting that VLPVPQK has the potential to be developed as a nutraceutical additive.

 Please wait while we load your content...
Please wait while we load your content...