A randomized, double-blind, placebo-controlled pilot study to assess bacterial anti-adhesive activity in human urine following consumption of a cranberry supplement†
Abstract
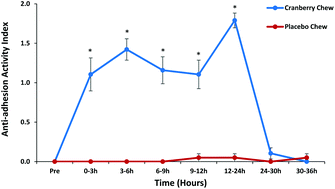
Urinary tract infections (UTIs) are one of the common bacterial infections treated with antibiotics. The North American cranberry is recommended for prophylaxis in women with recurrent UTIs as a nutritional alternative. The ability of cranberry components and their metabolites to inhibit adhesion of uropathogenic Escherichia coli (E. coli) is an important mechanism by which cranberry mitigates UTIs. The objective of this study was to evaluate urinary anti-adhesion activity against type 1 and P-type uropathogenic E. coli after consumption of cranberry +health™ cranberry supplement (cranberry chew). In this randomized, double-blind, placebo-controlled, crossover design pilot trial (n = 20), subjects consumed two cranberry or placebo chews, one in the morning and one in the evening. Clean-catch urine samples collected at the baseline and post-intervention (0–3, 3–6, 6–9, 9–12, 12–24, 24–30, 30–36 h) were tested for anti-adhesion effects with a mannose-resistant human red blood cell hemagglutination assay specific for P-type E. coli, or a T24 cell line model for type 1 E. coli. Urinary anti-adhesion activity against P-type E. coli after consumption of the cranberry chew was significantly greater (p < 0.05) than that observed with placebo chew at all time points except 24–36 h. Ex vivo anti-adhesion effects on type 1 E. coli were greater (p < 0.05) after cranberry chew consumption than placebo chew at 3–6 and 6–9 h urine collections. In conclusion, consumption of cranberry +health™ cranberry supplement exhibited greater ex vivo urinary anti-adhesion activity compared to placebo, suggesting that it may have the potential to help promote urinary tract health.

 Please wait while we load your content...
Please wait while we load your content...