Volumetric, acoustic, viscometric, calorimetric and spectroscopic studies to elucidate the effects of citrate and tartrate based food preservatives on the solvation behaviors of acidic amino acids at different temperatures†
Abstract
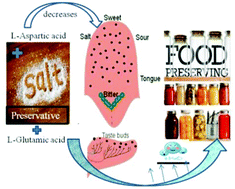
The effects of trisodium citrate (TSC) and disodium tartrate (DST) based food preservatives on the hydration behaviors of the amino acids L-aspartic acid (ASP) and L-glutamic acid (GLU) have been studied using thermodynamic, transport, calorimetric and spectroscopic studies. The volumetric, acoustic and viscosity data suggest that hydrophilic–ionic/hydrophilic interactions are predominant in these systems. The calculated parameters are worthwhile for exploring the solutes as structure-breakers, and the solutes undergo pairwise interactions with the co-solutes. The sweetness of both amino acids decreases in the presence of the preservatives. The hydration number and solvation data suggest that these solutes are more hydrated in water. The dominance of dehydration effects in relation to TSC is observed from the positive enthalpy changes in calorimetry studies and the negative chemical shifts in 1H NMR studies.


 Please wait while we load your content...
Please wait while we load your content...