Isolation and identification of iron-chelating peptides from casein hydrolysates
Abstract
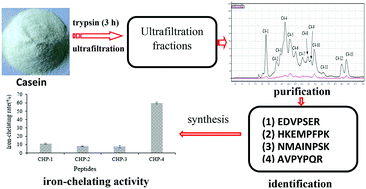
Iron deficiency is a common nutritional disorder worldwide. Peptides derived from protein hydrolysates have recently attracted interest as novel iron chelators due to their superiority in terms of increasing solubility, bioavailability, absorption and stability. The aim of this study was to isolate and identify iron-chelating peptides from casein hydrolysates. Casein was hydrolyzed (trypsin, 3 h) and subsequently isolated using ultrafiltration and RP-HPLC. Four iron-chelating casein hydrolysate peptides, named CHP-1, CHP-2, CHP-3 and CHP-4, were identified by LC-MS/MS, and their amino acid sequences were Glu-Asp-Val-Pro-Ser-Glu-Arg (EDVPSER), His-Lys-Glu-Met-Pro-Phe-Pro-Lys (HKEMPFPK), Asn-Met-Ala-Ile-Asn-Pro-Ser-Lys (NMAINPSK) and Ala-Val-Pro-Tyr-Pro-Gln-Arg (AVPYPQR), with molecular weights of 830.6120 Da, 1012.5280 Da, 873.4440 Da and 829.4570 Da, respectively. The artificially synthesized peptides of CHP-1, CHP-2, CHP-3 and CHP-4 were verified, and their iron-chelating rates were 11.14%, 8.02%, 7.57% and 59.76%, respectively. These results suggested that the isolated iron-chelating peptides might serve as potential iron supplements and be used as food additives and functional foods.


 Please wait while we load your content...
Please wait while we load your content...