Hydrogenation of substituted nitroaromatics on non-noble metal catalysts: mechanistic insights to improve selectivity
Abstract
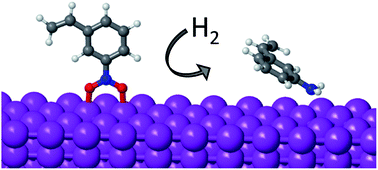
The mechanism of nitrobenzene hydrogenation on non-noble metals such as Ni is different from that previously reported for noble metals like Pt. The newly proposed pathway involves the initial dissociation of the two N–O bonds of nitrobenzene (Ph-NO2 → Ph-NO → Ph-N), leading to partial oxidation of the catalyst surface, followed by two successive hydrogenation steps (Ph-N → Ph-NH → Ph-NH2) that finally produce the functionalized aniline. Due to the oxophilic nature of non-noble metals like Ni, Co or Cu, the hydrogenation of the Ph-N intermediate and the removal of O in the form of water become the most energy demanding steps of the process. The strength of the interaction of O, H and N with different metals, and the preferential mode of adsorption of nitroarenes on clean and partially oxidized systems obtained from DFT calculations, are now used to propose an efficient non-noble metal catalyst that optimizes activity and selectivity.
- This article is part of the themed collection: Reaction mechanisms in catalysis


 Please wait while we load your content...
Please wait while we load your content...