Facile assembly of layer-interlocked graphene heterostructures as flexible electrodes for Li-ion batteries†
Abstract
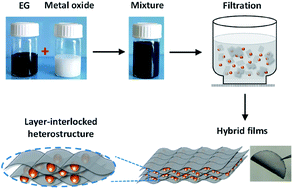
Flexible electrodes with robust mechanical properties and high electrochemical performance are of significance for the practical implementation of flexible batteries. Here we demonstrate a general and straightforward co-assembly approach to prepare flexible electrodes, where electrochemically exfoliated graphene (EG) is exploited as the film former/conducting matrix and different binary metal oxides (Li4Ti5O12, LiCoO2, Li2MnO4, LiFePO4) are incorporated. The resultant EG–metal oxide hybrids exhibit a unique layer-interlocked structure, where the metal oxide is conformably wrapped by the highly flexible graphene. Due to numerous contact interphases generated between EG and the intercalated material, the hybrid films show high flexibility and can endure rolling, bending, folding and even twisting. When serving as the anode for Li-ion batteries, the freestanding EG–Li4Ti5O12 hybrid presents a characteristic flat discharge plateau at 1.55 V (vs. Li/Li+), indicating transformation of Li4Ti5O12 to Li7Ti5O12. Small polarization, high rate capability and excellent cycling stability against mechanical bending are also demonstrated for the prepared EG–Li4Ti5O12 hybrid. Finally, full cells composed of EG–Li4Ti5O12 and EG–LiFePO4 hybrids show impressive cycling (98% capacity retention after 100 cycles at 1C) and rate performance (84% capacity retained at 2.5C). The straightforward co-assembly approach based on EG can be extended to other two-dimensional layered materials for constructing highly efficient flexible energy storage devices.
- This article is part of the themed collection: Chemistry of 2-dimensional materials: beyond graphene


 Please wait while we load your content...
Please wait while we load your content...