FBpin and its adducts and their role in catalytic borylations†
Abstract
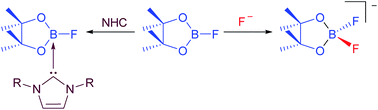
We present herein investigations concerning the reactivity of B2pin2 and FBpin (pin = pinacolato) with respect to fluoride ions and NHCs (NHC = N-Heterocyclic Carbene) in order to understand better the role of added fluoride in our recently reported Ni-catalyzed C–F borylation process, to confirm the nature of the NHC-adducts of FBpin, and to explain the formation of the species detected as side products of the defluoroborylation reaction. We report the calculated gas phase fluoride ion affinities (FIA) of relevant boron species and demonstrate that the presence of one equivalent of added F− in solution will bind and activate B2pin2 (FIA = +247 kJ mol−1), but it will also bind even more effectively to FBpin (FIA = +287 kJ mol−1) formed in the reaction. We prepared the NHC-adducts of FBpin by two different methods and isolated and characterized several examples of the type FBpin·NHC (NHC = iPr2Im, 1; Me2Im, 2; MeiPrIm, 3; nPr2Im, 4; Mes2Im 5). The salts [pinBF2][NMe4] 6, as well as the significantly more soluble analogue [pinBF2][NnBu4] 7, were also isolated and characterized, providing confirmation of the presence of the pinBF2 anion in various stoichiometric and catalytic borylation reactions.
- This article is part of the themed collection: Mechanistic processes in organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...