Suzuki–Miyaura coupling revisited: an integrated computational study†
Abstract
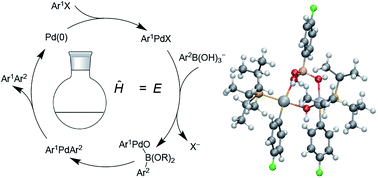
New density functional theory (DFT) computations are reported concerning the mechanism of the Suzuki–Miyaura cross-coupling reaction of aryl halides and aryl boronic acids catalyzed by palladium phosphine complexes. The calculations are aimed at refining the understanding of the overall catalytic mechanism using state of the art theoretical approaches (including, for the first time, an attempt to describe the Gibbs energy of the reactant base in a realistic way). New experimental results (Thomas and Denmark, Science, 2016, 352, 329–332) concerning pre-transmetallation intermediates with a Pd–O–B linkage provide an invaluable benchmark to test the accuracy of the calculations. The calculations show that bottlenecks to catalysis associated with oxidative addition, X-to-O substitution at Pd, and transmetallation can lie close in energy.
- This article is part of the themed collection: Mechanistic processes in organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...