Bambusurils as a mechanistic tool for probing anion effects†
Abstract
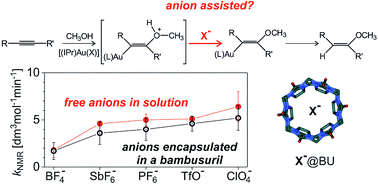
Bambusuril macrocycles have high affinity towards anions (X−) such as PF6− and SbF6− or BF4− and ClO4−. Therefore, addition of bambusurils to reaction mixtures containing these anions effectively removes the free anions from the reaction process. Hence, comparing reactions with and without addition of bambusurils can demonstrate whether the anions actively participate in the reaction mechanism or not. We show this approach for gold(I) mediated addition of methanol to an alkyne. The reaction mechanism can proceed via monoaurated intermediates (e.g., in catalysis with [(IPr)AuX]) or via diaurated intermediates (e.g., in catalysis with [(PPh3)AuX]). We show that anions X− slightly affect the reaction rates, however the effect stays almost the same even after their encapsulation in the cavity of bambusurils. We also demonstrate that X− affects the overall reaction rate in the very same way as the reaction rate of the protodeauration step. All results are consistent with the indirect effect of X− by the acidity of the conjugated acid HX on the rate-determining step. There is no evidence that a direct involvement of X− would affect the reaction rate.
- This article is part of the themed collection: Mechanistic processes in organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...