Investigations into the mechanism of copper-mediated Glaser–Hay couplings using electrochemical techniques†
Abstract
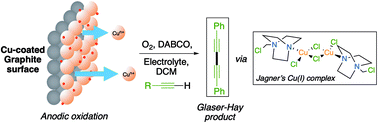
The mechanism of the copper mediated C–C bond forming reaction known as Glaser–Hay coupling (alkyne dimerization) has been investigated using electrochemical techniques. Applying an oxidative potential to a copper or copper-coated graphite electrode in the presence of the organic base DABCO results in the dimerization of phenylacetylene in good yield. Further mechanistic investigation has shown that this reaction medium results in the assembly of a dinuclear Cu(I) complex which, although previously reported, has never been shown to have catalytic properties for C–C bond formation. The complex is reminiscent of that proposed in the Bohlmann model for the Glaser–Hay reaction and as such lends weight to this proposed mechanism above the alternative proposed mononuclear catalytic cycle.
- This article is part of the themed collection: Mechanistic processes in organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...