Biopolymer monolith for protein purification
Abstract
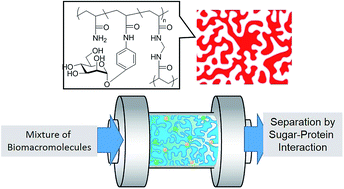
Porous glycopolymers, “glycomonoliths”, were prepared by radical polymerization based on polymerization-induced phase separation with an acrylamide derivative of α-mannose, acrylamide and cross-linker in order to investigate protein adsorption and separation. The porous structure was induced by a porogenic alcohol. The pore diameter and surface area were controlled by the type of alcohol. The protein adsorption was measured in both batch and continuous flow systems. The glycomonoliths showed specific interaction with the sugar recognition protein of concanavalin A, and non-specific interaction to other proteins was negligible. The amount of protein adsorption to the materials was determined by the sugar density and the composition of the glycomonoliths. Fundamental knowledge regarding the glycomonoliths for protein separation was obtained.
- This article is part of the themed collection: Nanolithography of biointerfaces


 Please wait while we load your content...
Please wait while we load your content...