Effect of alkali ions on optical properties of flavins: vibronic spectra of cryogenic M+lumiflavin complexes (M = Li–Cs)†
Abstract
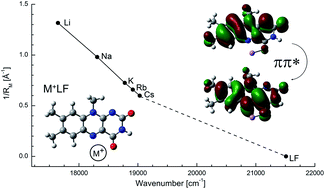
Flavin compounds are frequently used by nature in photochemical processes because of their unique optical properties which can be strongly modulated by the surrounding environment such as solvation or coordination with metal ions. Herein, we employ vibronic photodissociation spectroscopy of cryogenic M+LF complexes composed of lumiflavin (LF, C13H12N4O2), the parent molecule of the flavin family, and alkali ions (M = Li–Cs) to characterize the strong impact of metalation on the electronic properties of the LF chromophore. With the aid of time-dependent density functional theory calculations (PBE0/cc-pVDZ) coupled to multidimensional Franck–Condon simulations, the visible photodissociation (VISPD) spectra of M+LF ions recorded in the 500–570 nm range are assigned to the S1 ← S0 (ππ*) transitions into the first optically bright S1 state of the lowest-energy M+LF(O4+) isomers. In this O4+ structure, M+ binds in a bent chelate to the lone pairs of both the O4 and the N5 atom of LF. Charge reorganization induced by S1 excitation strongly enhances the interaction between M+ and LF at this binding site, leading to substantial red shifts in the S1 absorption of the order of 10–20% (e.g., from 465 nm in LF to 567 nm in Li+LF). This strong change in M+⋯LF interaction strength in M+LF(O4+) upon ππ* excitation can be rationalized by the orbitals involved in the S1 ← S0 transition and causes strong vibrational activity. In particular, progressions in the intermolecular bending and stretching modes provide an accurate measure of the strength of the M+⋯LF bond. In contrast to the experimentally identified O4+ ions, the predicted S1 origins of other low-energy M+LF isomers, O2+ and O2, are slightly blue-shifted from the S1 of LF, demonstrating that the electronic properties of metalated LF not only drastically change with the size of the metal ion but also with its binding site.
- This article is part of the themed collection: Advances in ion spectroscopy - from astrophysics to biology


 Please wait while we load your content...
Please wait while we load your content...