Ab initio phase diagrams of Hf–O, Zr–O and Y–O: a comparative study
Abstract
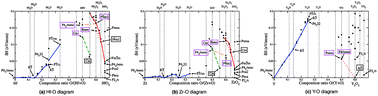
We present phase diagrams of binary oxides, Hf–O, Zr–O and Y–O, obtained by ab initio evolutionary simulations, in order to explore possible metastable crystalline suboxide structures which could be quenched during the electroforming processes within the conductive filaments in stoichiometric HfO2, ZrO2 and Y2O3 host materials, in resistive switching devices. We find that, in the range MO–MO2 (where M = Hf, Zr, Y), the most energetically favourable atomic configurations have properties which facilitate the ionic conduction of oxygen. Namely, the calculations reveal that oxygen vacancies tend to order in arrays of one-dimensional channels, along which the migration barrier of anions is much lower than for the stoichiometric hosts. We explore for Hf–O and Zr–O a new set of structural patterns, different from those of the host materials, for which a change of oxygen stoichiometry does not change the underlying structural frame. However, a sufficient change of oxygen stoichiometry drives metallic conductivity in oxygen-deficient compounds, whereas their oxygen-rich counterparts are insulating. In contrast to Hf–O and Zr–O, in the Y–O system we find an atomic structure with metallic conductivity, which has the same structural frame as the stoichiometric Y2O3 host. We believe that this property enables the forming-free resistive switching in Y2O3.
- This article is part of the themed collection: New memory paradigms: memristive phenomena and neuromorphic applications


 Please wait while we load your content...
Please wait while we load your content...