Characterizing colloidal metals in drinking water by field flow fractionation†
Abstract
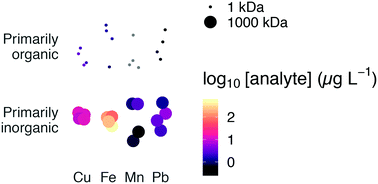
Exposure to lead, copper, and manganese via drinking water is a public health concern. Modeling can account for solubility due to simple inorganic ligands but generally falls short in capturing the effects of natural organic matter, particles, and colloids; environmental data are needed to understand the importance of these species. Here, we demonstrate the use of asymmetric flow field flow fractionation with UV and ICP-MS detection to separate drinking water samples into ionic species, colloids, and metal–organic complexes. Colloidal iron appeared to be more important as a transport vector for lead, copper, and manganese than natural organic matter: estimated colloidal metals concentrations (Pb, Cu, Fe, Mn >1000 kDa) were at least 3 times greater than concentrations of these metals associated with the high apparent molecular weight (∼1 kDa) organic signal. In transmission electron micrographs, two distinct populations of relatively dense colloids in the size range 50–200 nm were apparent, with morphologies typical of environmental iron oxides. Moreover, energy dispersive spectroscopy showed that sample colloids were rich in iron, copper, and oxygen. At equilibrium (e.g., during long stagnation periods in plumbing), high molecular weight organic and colloidal fractions represent additional capacity for drinking water to transport lead, which could have implications for water treatment to limit lead exposure.


 Please wait while we load your content...
Please wait while we load your content...