The reactivity and pathway of Fenton reactions driven by hydroxybenzoic acids: the effect of hydroxylation†
Abstract
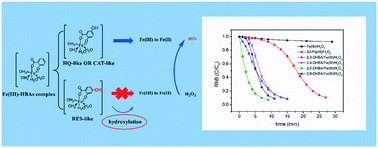
Hydroxybenzoic acids (HBAs) are neglected as promoters of the Fenton reaction due to their low reduction capacity of Fe(III) and facile capture of hydroxyl radicals. However, autocatalysis often occurs in HBA degradation by the Fe(III)/H2O2 system. In this study, we investigated the effect of various HBA compounds on the Fenton reaction, and found that model HBAs also showed good catalytic activity. Their capability to promote the Fenton reaction is closely related to their readiness to be transformed into a hydroquinone-like (HQ-like) species. Electron-donating groups (EDGs) can accelerate the hydroxylation of aromatic compounds, thus leading to the rapid formation of a HQ-like species.
- This article is part of the themed collection: Best Papers 2019 – Environmental Science: Water Research & Technology


 Please wait while we load your content...
Please wait while we load your content...