Natural organic activator quercetin for persulfate oxidative degradation of halogenated hydrocarbons†
Abstract
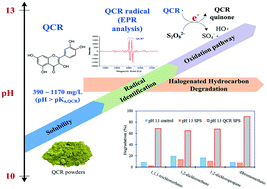
Organic activation is a method that can be used to generate SO4−˙ from persulfate (PS). Polyphenols contain phenolic functional groups and can be divided into five categories; one of these categories, the flavonoids, has structures that cause a lack of electron delocalization and allow the release of electrons for activating PS. A survey of numerous flavonoids indicated that quercetin (QCR) with relatively high antioxidant activity and low cost may be worth investigating as a PS activator. This study initially evaluated QCR's solubility at different pH values and found it to be 9–11 mg L−1 at pH 1–9 and 394–1171 mg L−1 at pH 10–13. Considering QCR's solubility range, further experiments were performed to investigate the degradation of 14 halogenated hydrocarbons by QCR activated PS at 20 °C, using 1.0 mM QCR and 20 mM PS at pH 13. The study indicated that the PS only and PS/QCR systems at pH 13 resulted in nearly complete degradation of 5 compounds. The PS only system at pH 7 and 13 exhibited somewhat similar degradation results. However, it was noticeable that 1,1-dichloroethane, 1,2-dichloroethane (1,2-DCA), 1,2-dichloropropane and dibromomethane cannot be easily degraded by PS at pH 7 and 13, but are effectively degraded by PS/QCR at pH 13. Consequently, 1,2-DCA was selected to examine its degradation kinetics. Electron paramagnetic resonance analysis indicates that QCR radicals were formed at pH 13 and could release electrons for PS activation upon stabilization to form benzoquinones. SO4−˙ and HO˙ were mainly detected in the QCR/PS pH 13 system.


 Please wait while we load your content...
Please wait while we load your content...