Soft electrodes in water desalination: application to multi-valent ions
Abstract
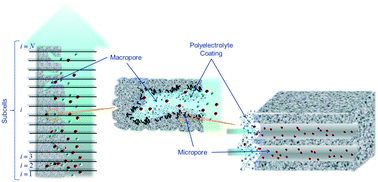
The capacitive deionization (CDI) method, in which the capacitance of the electrical double layers is used for removing ions from aqueous solutions, can be more efficient if some procedures are devised to help the bare electrode double layers in adsorbing ions. One of these methods is based on the use of ion-selective membranes (membrane-CDI or MCDI). An alternative has been proposed, in which the electrodes are coated with a polyelectrolyte layer, also of suitable polarity. The method has been called soft electrode CDI or SECDI. In this work, we examine how it can be applied to a mixed solution containing divalent and monovalent cations, specifically, sodium and magnesium, identifying to what extent each of them is removed from the solution. A model is presented in which the polyelectrolyte is assumed to coat the macropore walls, and hence the mouths of the micropores connected to them. The results of the model, corroborated by experimental determinations of SECDI in mixed solutions of NaCl and MgCl2, demonstrate that magnesium, even if in tiny amounts, dominates the deionization with soft electrodes, as it is more abundant in the polyelectrolyte layer than sodium.
- This article is part of the themed collection: Interfaces Against Pollution


 Please wait while we load your content...
Please wait while we load your content...