Modeling effects of H2S on electron competition among nitrogen oxide reduction and N2O accumulation during denitrification†
Abstract
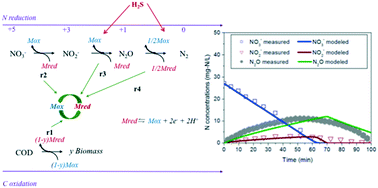
Nitrous oxide (N2O) accumulation during heterotrophic denitrification can be severely affected by hydrogen sulfide (H2S) that is generated from sewer networks and introduced to the denitrification system. In this study, a new mathematical model was developed to assess nitrogen oxide dynamics during heterotrophic denitrification in the presence of different H2S concentrations. The model describes electron competition during three-step denitrification (i.e., nitrate to nitrite, nitrite to N2O, and N2O and nitrogen gas) through linking nitrogen reduction and carbon oxidation processes with a pool of electron carriers and considering a non-competitive H2S inhibition function on nitrite and N2O reduction kinetics. The developed model well reproduced experimental nitrogen oxide data caused by the H2S inhibition on denitrification with methanol-utilizing denitrifying cultures under different operational scenarios, indicating model validity and applicability. Modeling results revealed that N2O accumulation could be increased with elevated H2S levels, due to the decreased electron competition capacity of N2O reduction compared to that of nitrate and nitrite reduction. Increasing electrons were distributed to nitrate and nitrite reduction due to their higher tolerance to H2S. Less N2O accumulation can be achieved by extending the feeding period of H2S.


 Please wait while we load your content...
Please wait while we load your content...