Pyridine-grafted Cr-based metal–organic frameworks for adsorption and removal of microcystin-LR from aqueous solution†
Abstract
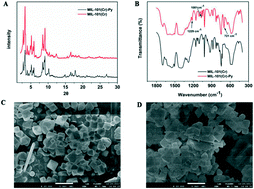
A Cr-based metal–organic framework grafted with pyridine was named MIL-101(Cr)-Py, and was used to adsorb microcystin-LR (MC-LR) from aqueous solution. The adsorption process and mechanism were investigated. The adsorption kinetics and isotherms obeyed the pseudo-second-order kinetics and Langmuir adsorption model, respectively. MIL-101(Cr)-Py exhibits rapid adsorption in 30 min at 25 °C. Due to the introduction of the pyridine group on MIL-101(Cr), the maximum adsorption capacity of MIL-101(Cr)-Py (Q0 = 409.8 mg g−1) is higher than that of virgin MIL-101(Cr) at 25 °C. The adsorption thermodynamics show that the adsorption of MC-LR on MIL-101(Cr)-Py could be entropy-driven. Moreover, the remarkable performance of MC-LR in adsorption may be due to the electrostatic interaction, π–π stacking interaction, and dipole–dipole interaction between MC-LR and MIL-101(Cr)-Py. Therefore, the inherent advantages of MIL-101(Cr)-Py such as facile modification, high adsorption capacity, and rapid adsorption may pave the way towards highly-efficient MC-LR removal in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...