Degradation of ciprofloxacin by 185/254 nm vacuum ultraviolet: kinetics, mechanism and toxicology†
Abstract
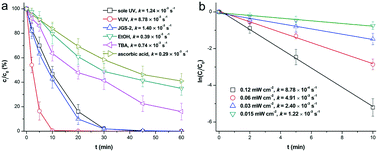
Fluoroquinolone antibiotics (FQs) are highly resistant to conventional biological water treatment processes, thus, it is desirable to develop novel water treatment methods for eliminating FQs efficiently. Vacuum ultraviolet (VUV; 185/254 nm) may be a novel alternative due to its capacity of photolyzing water into hydroxyl radicals without chemical addition. Ciprofloxacin (CIP) was selected as the representative of FQs, and its degradation using VUV treatment was tested. A first-order reaction kinetics with a rate constant (kapp) at 8.78 × 10−3 s−1 was observed when [CIP]0 = 3.02 μM and 185 nm irradiation intensity = 0.12 mW cm−2. Wavelength screening, radical scavenging and kinetic calculations revealed that direct photolysis and indirect radical-based oxidation both contributed to the degradation, with a reaction rate constant (k˙OH–CIP) at (8.6 ± 0.8) × 109 M−1 s−1. Radical-based addition, substitution and cleavage of CIP generated five series of stable products. Most products in the early stage were simple hydroxylated intermediates from CIP. Based on the toxicology analysis including reactive oxygen species and apoptosis of Escherichia coli, these early-stage products were supposed to have higher toxicity than CIP. However, as they were transformed into further oxidized products, their toxicity was reduced. Variable pH values and natural organic matter/anions significantly affected the degradation efficiency. Altogether, VUV induced the rapid degradation of CIP, and it will be a promising treatment method for transforming and detoxifying micro-pollutants from water.


 Please wait while we load your content...
Please wait while we load your content...