Coupled morphological and structural evolution of δ-MnO2 to α-MnO2 through multistage oriented assembly processes: the role of Mn(iii)†
Abstract
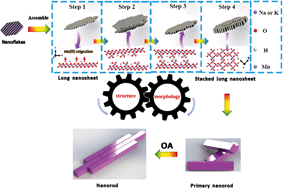
α-MnO2 is a typical tunneled Mn oxide (TMO) that is frequently associated with δ-MnO2 in the environment and exhibits strong adsorption and oxidation activity. The morphology of α-MnO2, which is controlled by an oriented attachment (OA) process, is one of the key factors affecting its reactivity. However, the detailed crystal growth process and coupling between morphology and structure of α-MnO2 during OA processes remain poorly understood. We propose that the transformation of layer-based δ-MnO2 to tunnel-based α-MnO2 occurs via a multistage OA process. In the initial stage, the produced δ-MnO2 nanoflakes are found to spontaneously self-assemble into a nanoribbon with a large number of lattice defects via edge-to-edge OA. The presence of lattice defects promotes the generation of oxygen vacancies, and the Mn(IV) ions in the [MnO6] octahedral layers of δ-MnO2 are reduced to Mn(III)/Mn(II). The reduced ions subsequently migrate from the [MnO6] octahedral layers to the interlayers during this process. The hydroxide which acts in coordination with the interlayer Mn(III)/Mn(II) and oxygen atoms coordination with adjacent nanoribbons attach to each other driven by hydrogen bonding and form primary nanorods through a face-to-face OA mechanism along the c-axis. Concomitantly, the bonding of [Mn(III)O6] octahedra in the interlayer of the nanoribbons with adjacent [MnO6] octahedral layers leads to the fabrication of a new α-MnO2 tunnel structure from the original δ-MnO2. These findings provide insights into both the transformation mechanisms of the layer-based to the tunnel-based nanoparticles and methods for the efficient and controlled synthesis of nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...
