Reactivity of graphene oxide with reactive oxygen species (hydroxyl radical, singlet oxygen, and superoxide anion)†
Abstract
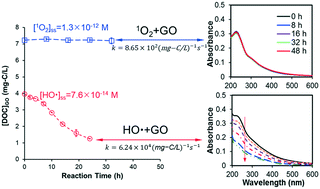
Increases in the production and applications of graphene oxide (GO), coupled with reports of its toxic effects, are raising concerns about its health and ecological risks. To better understand GO's fate and transport in aquatic environments, we investigated its reactivity with three major reactive oxygen species (ROS): HO˙, 1O2, and O2˙−. Second-order degradation rate constants were calculated on the loss of dissolved organic carbon (DOC) and steady-state concentration of individual ROS species. Absolute second-order rate constants were determined by competition kinetics to be 6.24 × 104, 8.65 × 102, and 0.108 mg-C−1 L s−1 for HO˙, 1O2, and O2˙−, respectively. Photoreduced GO products had a similar reactivity to HO˙ as GO, with rate constants comparable to polycyclic aromatic compounds, but about two times higher than dissolved organic matter on a per carbon basis. Reaction with HO˙ resulted in decomposition of GO, with loss of color and formation of photoluminescent products. In contrast, reaction with 1O2 showed no effect on DOC, UV-vis spectra or particle size, while reaction with O2˙− slightly reduced GO. These results demonstrate that interactions with ROS will affect GO's persistence in water and should be considered in exposure assessment or environmental application of GO.


 Please wait while we load your content...
Please wait while we load your content...