Ferric reducing reactivity assay with theoretical kinetic modeling uncovers electron transfer schemes of metallic-nanoparticle-mediated redox in water solutions†
Abstract
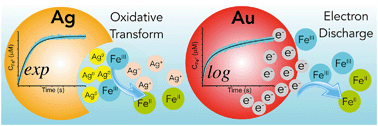
Metallic nanoparticles (NPs) can mediate electron transfer in water solutions. We report a chemical assay named “ferric reducing ability of nanoparticles” (FRAN), which is capable of probing electron fluxes at NP–water interfaces. The assay provides time-resolved colorimetric analysis for evaluating the surface reactivity of metallic NPs in redox processes and in elucidating the electron transfer schemes. FRAN includes the reduction of ferric (FeIII) to ferrous (FeII) ions by electrons transferred from NPs at the NP–water interface. In tests of 20 nm gold (Au) and silver (Ag) NPs, the plateaued FeII concentration after adequate reaction time was linearly correlated to the NP concentration. However, distinct kinetic trends, which are mathematically described by an exponential and logarithmic function, were observed between Ag and Au NPs. We developed two theoretical models, which correspond to two interfacial electron transfer schemes, to explain the difference between the materials. For Ag NPs, the occurring redox is a one-electron transfer from Ag0 to FeIII, leading to formation of Ag+ and FeII. Despite being a heterogeneous reaction including the NP solid and aqueous solution, it involves electron transfer at the interfacial region in which the collision between FeIII and Ag0 occurs in a approximately homogeneous manner, and thus its kinetics behaves as a pseudo first-order reaction. For Au NPs, an electron flux forms when electrons stored in the NP phase are “discharged” to reduce FelII at the interface, and the NPs act as electrodes. The experiments and models for FRAN can aid in the design of NP antioxidant functionality and assessment of environmental impacts of NPs in water or other fluids.


 Please wait while we load your content...
Please wait while we load your content...
